Compound Boggle
Grade Level: Grades 11 and 12
Description
Students compete to name as many compounds as possible in a random array of elements.
Participants
Teams of up to six.
The school must provide a teacher at the event to help with scoring of the rounds in which his/her school is competing.
Schedule
Teams will be assigned to one of four (4) time slots. In each time slot there will be two rounds of Compound Boggle. After the first boggle, half the teams will be eliminated based on score.
The top team from each time slot will advance to the final round of competition to determine first, second, and third places.
Time slots
- 9:15 AM - 9:55 AM
- 10:00 AM - 10:40 AM
- 10:45 AM - 11:25 AM
- 11:30 AM - 12:10 PM
Finals begins at 12:20 PM
Materials
Teams will receive the same randomly created 4 x 4 array of element symbols. They will be given 10 minutes to locate and write the formulae of as many inorganic compounds as they can using any sequence of horizontally, vertically, or diagonally adjacent elements.
Instructions
- Teams will be asigned to one of four time slots. Approximately seven teams will compete in each time slot.
- Teams will assemble around a seat on one side of an auditorium. The only aid allowed will be a simple periodic table of elements containing only element symbols. No pencils, pens, or paper may be used by the teams at this Array Seat. The Contest Array may be used at this location only.
- On the other side of the auditorium will be an Entry Seat. Only one team member at a time may go to this seat. That student may write only one formula and name, located in the array by his team on the paper provided. This entry will be covered by the school file folder and may not be changed or even looked at after the entry has been completed. In tag team fashion, other members of the team will run to the Entry Seat, write a formula and name, and return. The entire team need not participate in the entry of substances.
- No communication other than encouragement is allowed between the team at the Array Seat and the student at the Entry Seat.
- At the end of the contest, any student at the Entry Seat, in the process of writing a substance, may complete that entry.
- Each team will complete two arrays. The three teams with the highest cumulative score, regardless of timeslot, will advance to the final where they will compete by completing one more array.
Rules
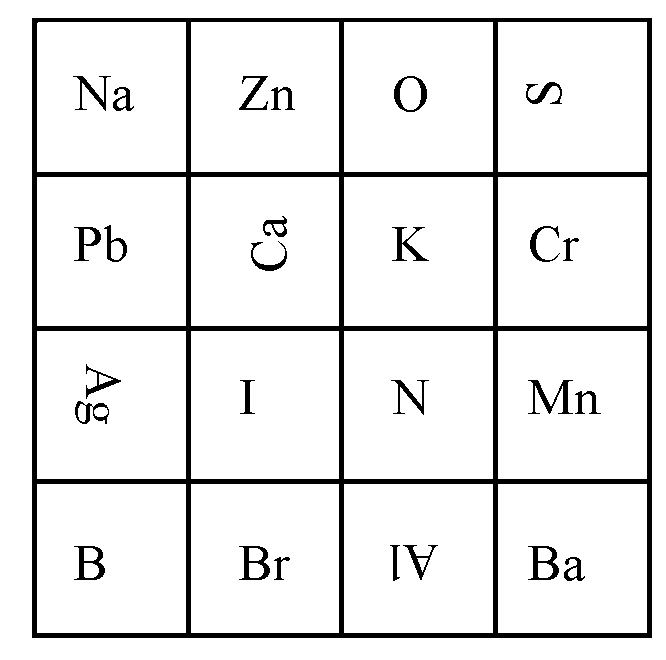
- The goal is to enter as many inorganic compounds that can be completed from the array as possible
- Similar to the game ‘Boggle’, competitors may make compounds from any order of elements on the array that are touching and in a sequential order that matches the compound. Elements may connect horizontally, vertically, or diagonally.
- All answers must obey the rules for inorganic naming. This means you must have an element with a positive oxidative state first combined with an element or polyatomic ion with a negative oxidative state. Organic names such as methane are not allowed.
- All answers must have a unique order of elements. For example, sulfur dioxide (SO2) is possible since sulfur has a +4 oxidative state and oxygen has a -2 oxidative state. Another possible answer would be sulfur trioxide (SO3) since sulfur also has a +6 oxidative state. However, if both of these answers were used, one of them would be marked incorrect since they both employ the same order of elements
However, hydrogen and chlorine offer an interesting opportunity. Since both hydrogen and chlorine have both a +1 and a -1 oxidative state, both hydrogen chloride (HCl) and monochlorine hydride (ClH) can be used since the order of elements is different. Of course monochlorine hydride has no practical meaning in the real world, but it correctly obeys the rules of compound boggle. - The compound name must unambiguously give the correct formula. For example, Hydrogen sulfide has only one possible compound: H2S. Dihydrogen sulfide is also correct, but since hydrogen only has a +1 oxidative state, and sulfur’s only negative oxidative state is -2, then no other combination is possible.
- You may NOT form any compound by combining a positive oxidation state of a non-metallic element with ANY polyatomic negative ions such as chlorate (ClO31-) or sulfate (SO42-) etc. For example, Sulfur carbonate (SCO3) would be incorrect. For the purposes of this game, elements with a negative oxidative state are classified as non-metallic, with the exception of hydrogen. In addition, Boron and Silicon are classified as non-metallic (even though they do not exhibit a negative oxidative state).
Allowed Elements
While any element is in principle allowable, most of the ARRAY will consist of the following elements:
| H | Li | Na | K | Be | Mg |
| Ca | Ba | Cr | Mn | Fe | |
| Cu | Ag | Zn | Hg | B | |
| Al | C | Si | Pb | Sn | |
| N | P | As | Sb | O | |
| S | F | Cl | Br | I |
Periodic Table
The only allowable oxidation states are those published in the Official Compound Boggle Periodic Table. Note that the oxidation state of -1 is permitted for hydrogen in the formation of "hydrides".
Polyatomic Ions
The following list is provided of the ONLY POLYATOMIC IONS that will be accepted during the event. If we have missed a favourite of yours, please submit the ion, its formula and proper name in writing and we will keep it in mind for next year! Please notice that while persulfate, sulphate, sulfite and hyposulfite are very interesting, they repeat the same "SO" sequence and according to the rules there is no advantage in using "derivative" ions over the basic sulphate ion in forming compounds during the contest.
| AlO 2 ^ -1 | aluminate |
| C 2H 3O 2 ^ -1 | acetate |
| NH 4 ^ +1 | ammonium (ion) |
| AsO 4 ^ -3 | arsenate |
| BrO 3 ^ -1 | bromate |
| CO 3 ^ -2 | carbonate |
| ClO 3 ^ -1 | chlorate |
| CrO 4 ^ -2 | chromate |
| CNO ^ -1 | cyanate |
| CN ^ -1 | cyanide |
| HCO 3 ^ -1 | hydrogen carbonate |
| HS ^ -1 | hydrogen sulfide ion |
| HSO 4 ^-1 | hydrogen sulphate |
| OH ^ -1 | hydroxide |
| IO 3 ^ -1 | iodate |
| HPO 4 ^ -2 | monohydrogen phosphate |
| NO 3 ^ -1 | nitrate |
| MnO 4 ^ -1 | permanganate |
| PO 4 ^ -3 | phosphate |
| SeO 4 ^ -2 | selenate |
| SiO 3 ^ -2 | silicate |
| SO 4 ^ -2 | sulphate |
| SCN ^ -1 | thiocyanate |
Scoring
- One point will be AWARDED for each combination of correct formula and name entered.
- One point will be DEDUCTED for any error in EITHER the formula or name entered.
- One point will be DEDUCTED for any REPEATED formula and name combination in the team's entry. This includes the type of repeat identified in rule 4 above.
- Should the above rules result in any controversy, the decision of the Judges will be final.
Judging
Teams will be assigned to one of four time slots. In each time slot, there will be two rounds of Compound Boggle. The team score is the sum of the two scores.
The top three teams, regardless of timeslot, will participate in the final round of the competition to determine the first, second and third places.
FAQ
Rule #5 states that you can NOT form any compound by combination of a positive oxidation state of a non-metallic element with ANY polyatomic negative ion, so would it not be possible to answer HBrO3 (bromic acid) or any acid that involves a polyatomic ion such as carbonic acid?
Since hydrogen is in group one it is classified as a member of the alkali metal family, with general configuration 's 1' Note that hydrogen forms covalent compounds with nonmetals and ionic compounds with metals (hydride, H -1) like the nonmetals do. Since hydrogen bonded to nonmetals will ionize to form acids, HCl can be called hydrogen chloride or its (ionized) acid name hydrochloric acid. Also that since polyatomic ions contain covalent bonds within them, and since something like pure H 2SO 4 is actually fully covalently bonded until it ionizes in aqueous solution we can call the compound hydrogen sulfate or sulfuric acid. I tell students 'hydrogen is a group one element that behaves like a nonmetal'. Since it is grouped with metals, it can form compounds as metals do, and it can also behave as a nonmetal. Keep in mind also that antimony is classified as a nonmetal for the purpose of the game because it exhibits a negative oxidation state. I realize that the same can be said for hydrogen, though we are classifying hydrogen as a metal when it comes to polyatomic ions. I tell my students that chemistry has lots of rules, and then it has all of the exceptions to those rules. This classifies the study of chemistry as 'a form of entertainment'. Why do we have exceptions to the rules? Because it's fun!
We noticed in the Merck Index that bromic acid can also be written as BrHO 3 , if the elements appeared in that order on the grid could it be written in that form?
I think it's pretty rare to see bromic acid written BrHO 3 however the permitted list of polyatomic ions has the bromate ion as BrO 3 -1 so this ion can be used when the elements connect in the right order on the grid. The permitted polyatomic ions can be used when the elements occur in the grid in the order they are written on the permitted list. You are probably aware that the reason why sulfate is allowed but not sulfite or thiosulfate is because all three ions use the same sequence of elements, so placing only one ion on the list speeds up the marking process for the judges because the same sequence of elements may not be repeated on the answer sheet. So the only polyatomic ions permitted are the ones on the list, written in the order they appear on the list. For any compounds containing sulfur, we allow 'f' or 'ph' in the spelling. Teams know that writing 'f' is faster!
How are metalloids such as boron being treated? Can we form compounds such as boron chlorate?
Boron has always been classified as a nonmetal for this game, even though it exhibits only a +3 oxidation state. Nonmetals may not be combined with polyatomic ions. Si (also only +4, +2), As, Te, At are nonmetals. Al, Ge, Po are metals. Antimony has always been classified as a nonmetal in the game because it exhibits a negative oxidation state ('antimonide'). Note that hydrogen bonds like a nonmetal, forming covalent compounds with other nonmetals and forming ionic compounds with metals (hydrides). Hydrogen can combine with polyatomic ions to form the oxyacids (sulfuric, nitric, etc.).

